Medical device sterilization methods
Medical device sterilization methods
The Importance of Medical Device Sterilization
Medical device sterilization is a crucial requirement for any device used in a medical procedure, from stethoscopes used for routine checkups to scalpels used for surgeries. Sterilization kills microorganisms on the surface of devices. When devices are not properly sterilized, infectious diseases can spread during procedures, which can lead to illness or even death for patients.
When new medical devices are designed, scientists think carefully about how they will be sterilized. Design flaws in medical devices which prevent them from being properly sterilized can be dire. For example, in 20151 there was an outbreak of antibiotic-resistant bacterial infections which spread because of a design problem in duodenoscopies used to access the small intestine. The design mistake meant that the devices could not be properly sanitized, resulting in 400 people being infected with the antibiotic-resistant bacteria and leading to at least 35 deaths.
Why Different Devices Require Different Sterilization Methods
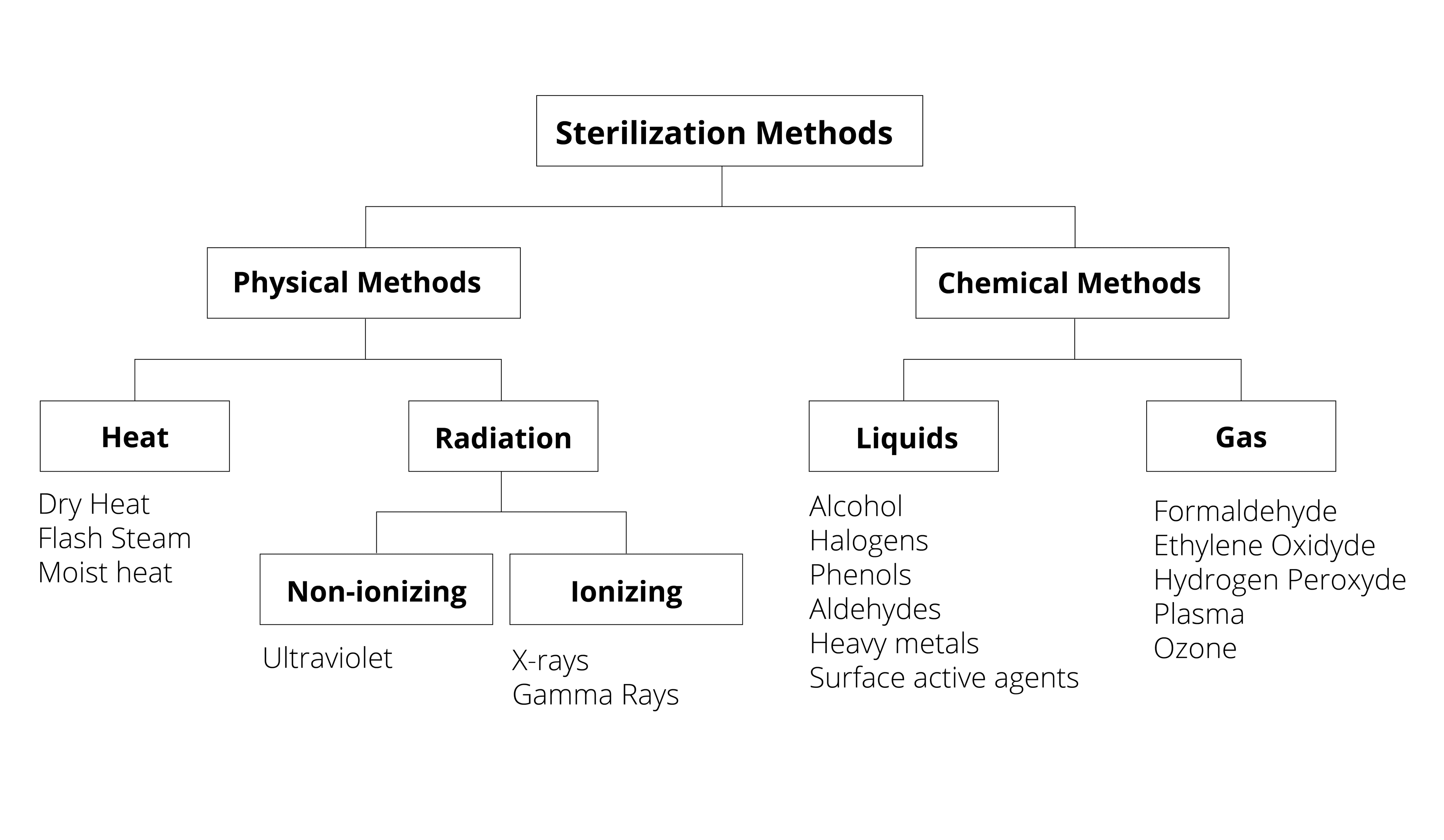
Sterilization processes depend on the type of device being sterilized–its intended use, the materials it is made of, its geometry, and other factors. The majority of medical and surgical devices used in healthcare are made of heat-stable materials. This means that they can be sterilized with heat, usually in the form of steam. However, over the past 70 years, medical devices have been developed which require more sophisticated sterilization methods. This is because they are made of materials which are sensitive to moisture and heat, such as plastic.2Determining the correct sterilization method for a device is important for ensuring that microorganisms are neutralized and that the device is not damaged in the sterilization process.
An Overview of Common Sterilization Methods:
Steam Sterilization: This method of sterilization, where an item is exposed to direct contact with steam at a specific temperature and pressure, is the most widely used sterilization technique. It is inexpensive and nontoxic. It can cause some damage to medical devices over time. However, this is the case with almost any sterilization process, which is why sterilization processes are validated before a product comes to market.3
Flash Sterilization: This is a modified version of conventional steam sterilization where the item being sterilized is put in an open tray or other special container to allow for rapid steam penetration.4
Ethylene Oxide: or EO is a low-temperature sterilization method which has been used since the 1950s for devices which are sensitive to moisture and temperature. Recently, there has been misinformation spread about this method of sterilization saying that it causes cancer, but these claims are not factual. EO gas is a safe and effective sterilization method. 5
Peracetic Acid is a biocidal oxidizer. It can be used by an automated machine to chemically serialize sensitive instruments like endoscopes and arthroscopes.6
Hydrogen Peroxide Gas Plasma: is a technology which was patented in 1987. Gas plasmas are created in enclosed chambers using a vacuum. Radio frequencies or microwave energies are then used to excite the gas molecules in the chamber and create what are called “free radicals,” or atoms with an unpaired electron. Free radicals are very reactive and can disrupt the metabolism of microorganisms.6
Gamma Irradiation: This sterilization method involves exposing a medical device to a source of radiation, usually Cobalt 60 isotope. This decomposes into Nickel 60 isotope and releases gamma rays which deactivate microorganisms that could be present on the device.7
How Medical Device Makers Ensure Their Products Are Sterile

Sterilization is important for devices that are used multiple times for different patients. It is equally crucial for disposable medical supplies such as syringes, scalpels, and swabs. Impurities which may have come into contact with these devices during the manufacturing process need to be removed.8 At OPT, we, along with our partners, perform quality and safety checks at each step of our manufacturing, sterilizing, and delivery process. For our InstaSwabTM family of products, we utilize the EO gas method outlined above. A crucial step in creating world-class medical devices is using world-class sanitation procedures to ensure they are safe.
Bibliography
- Hand, Sarah. “How to Choose the Best Medical Device Sterilization Method.” Xtalks, 17 Mar.2021, https://xtalks.com/how-to-choose-the-best-medical-device-sterilization-method/
- Center for Devices and Radiological Health. “Ethylene Oxide Sterilization for Medical Devices.” U.S. Food and Drug Administration, FDA, https://www.fda.gov/medical-devices/general-hospital-devices-and-supplies/ethylene-oxide-sterilization-medical-devices
- “Sterilization.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 18 Sept. 2016, https://www.cdc.gov/infectioncontrol/guidelines/disinfection/sterilization/index.html.
- “Sterilization.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 18 Sept. 2016, https://www.cdc.gov/infectioncontrol/guidelines/disinfection/sterilization/index.html.
- “Sterilization.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 18 Sept. 2016, https://www.cdc.gov/infectioncontrol/guidelines/disinfection/sterilization/index.html.
- “Sterilization.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 18 Sept. 2016, https://www.cdc.gov/infectioncontrol/guidelines/disinfection/sterilization/index.html.
- Charles Garcia,“Gamma Irradiation Sterilization.” StarFish Medical, 16 July 2021, https://starfishmedical.com/blog/gamma-irradiation-sterilization/.
- “Nasal Swab Protocols.” National Institutes of Health, U.S. Department of Health and Human Services, https://3dprint.nih.gov/collections/covid-19-response/nasal-swabs/protocols.